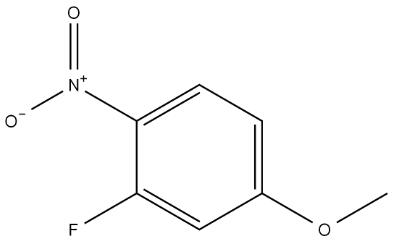
The Synthesis and Safety Considerations of 3-Fluoro-4-nitroanisole
2024-08-13
3-Fluoro-4-nitroanisole is a key intermediate used in the synthesis of various organic compounds, particularly in the pharmaceutical and agrochemical industries. While its applications are diverse, the synthesis and handling of this compound require careful attention to safety and environmental considerations. In this blog, we will explore the methods used to synthesize 3-Fluoro-4-nitroanisole, as well as the safety precautions necessary when working with this compound.
Synthesis of 3-Fluoro-4-nitroanisole
The synthesis of 3-Fluoro-4-nitroanisole typically involves the nitration of 3-fluoroanisole, followed by purification processes to isolate the desired product. Here’s a general overview of the synthetic pathway:
1. Starting Material: 3-Fluoroanisole
- The synthesis begins with 3-fluoroanisole, an aromatic compound where the fluorine atom is already attached to the benzene ring. This compound serves as the foundation for further chemical modifications.
2. Nitration Reaction:
- The key step in the synthesis is the nitration of 3-fluoroanisole. This reaction is usually carried out using a mixture of concentrated nitric acid (HNO3) and sulfuric acid (H2SO4). The nitration process introduces a nitro group (-NO2) at the para position relative to the methoxy group (-OCH3), forming 3-Fluoro-4-nitroanisole.
3. Purification:
- After the nitration reaction, the product is typically purified through recrystallization or column chromatography to obtain pure 3-Fluoro-4-nitroanisole. The purity of the compound is crucial for its use in further chemical reactions and applications.
4. Quality Control:
- The final product undergoes quality control tests to ensure it meets the required specifications. Techniques such as NMR spectroscopy, mass spectrometry, and HPLC (High-Performance Liquid Chromatography) are commonly used to verify the identity and purity of the compound.
Safety Considerations
Working with 3-Fluoro-4-nitroanisole requires careful attention to safety due to the potential hazards associated with its synthesis and handling. Here are some key safety considerations:
1. Chemical Hazards:
- 3-Fluoro-4-nitroanisole is classified as a hazardous substance. It may cause irritation to the skin, eyes, and respiratory system upon contact or inhalation. Proper personal protective equipment (PPE) such as gloves, goggles, and lab coats should be worn when handling the compound.
2. Handling and Storage:
- The compound should be handled in a well-ventilated area, preferably in a fume hood, to avoid exposure to vapors or dust. It should be stored in a cool, dry place, away from incompatible substances like strong acids or bases. Proper labeling and secure storage are essential to prevent accidental exposure or spills.
3. Environmental Impact:
- The synthesis of 3-Fluoro-4-nitroanisole involves the use of strong acids and other hazardous chemicals, which can have an environmental impact if not managed properly. Waste disposal should be conducted in accordance with local regulations, and efforts should be made to minimize the generation of hazardous waste during the synthesis process.
4. Emergency Procedures:
- In case of accidental exposure or spills, emergency procedures should be in place. This includes having access to safety showers, eyewash stations, and first aid kits. Workers should be trained in proper emergency response protocols to mitigate the effects of exposure.
Applications in Industry
Despite the safety considerations, the benefits of 3-Fluoro-4-nitroanisole in chemical synthesis cannot be overlooked. Its role as an intermediate in the production of pharmaceuticals and agrochemicals makes it a valuable compound in various industries. By adhering to safety guidelines and best practices, chemists can safely utilize this compound to develop new and innovative products.
Conclusion
3-Fluoro-4-nitroanisole is a crucial intermediate in organic synthesis, with applications spanning pharmaceuticals, agrochemicals, and beyond. Understanding the synthesis process and safety considerations is essential for anyone working with this compound. By prioritizing safety and environmental responsibility, the chemical industry can continue to harness the potential of 3-Fluoro-4-nitroanisole while minimizing risks to workers and the environment.